Multiple Choice
Which of the following gives the balanced equation for this reaction? 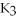
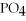 + Ca
+ Ca 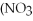
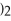 →
→ 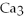
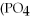
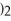 +
+ 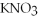
A) 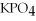 +
+ 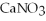 +
+ 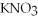
B) 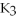
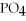 + Ca
+ Ca 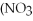
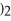 →
→ 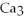
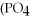
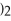 + 3
+ 3 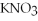
C) 2 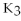
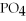 + Ca
+ Ca 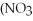
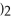 →
→ 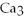
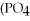
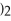 + 6
+ 6 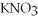
D) 2 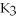
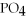 + 3Ca
+ 3Ca 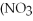
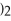 →
→ 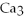
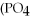
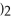 + 6
+ 6 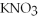
E) 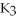
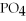 + Ca
+ Ca 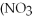
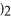 →
→ 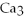
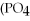
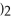 +
+ 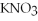
Correct Answer:

Verified
Correct Answer:
Verified
Q45: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen
Q64: Answer the question(s)below about the following reaction.<br>2
Q80: Answer the question(s)below about the following reaction.<br>2
Q84: 0.400 mole of sucrose, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="0.400
Q85: For the following question(s),consider the following balanced
Q86: In the following reaction Al is oxidized.<br>2Al
Q87: When the following equation is balanced,the coefficient
Q89: When the following equation is balanced,the coefficient
Q90: One mole of helium gas weighs _.<br>A)1.000
Q92: Answer the question(s)below about the following reaction.<br>2