Multiple Choice
Answer the question(s) below about the following reaction.
2 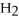
 → 2
→ 2 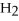 O +
O + 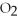
-How many moles of hydrogen peroxide (H2O2) are needed to produce 5.0 moles of water?
A) 1.0 mole
B) 2.0 moles
C) 2.5 moles
D) 4.0 moles
E) 5.0 moles
Correct Answer:

Verified
Correct Answer:
Verified
Q41: In an oxidation-reduction reaction,the substance reduced always
Q45: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen
Q64: Answer the question(s)below about the following reaction.<br>2
Q87: When the following equation is balanced,the coefficient
Q88: Which of the following gives the balanced
Q89: When the following equation is balanced,the coefficient
Q90: One mole of helium gas weighs _.<br>A)1.000
Q94: For the following question(s),consider the following equation.<br>2Mg
Q95: For the following question(s),consider the following balanced
Q97: How many moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="How