Multiple Choice
For the following question(s) ,consider the following balanced equation. 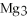
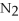 + 6
+ 6 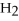 O → 3
O → 3 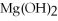 + 2
+ 2 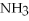
-When 36.0 g of 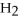 O react,how many grams of
O react,how many grams of 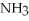 are produced?
are produced?
A) 34.0 g
B) 10.0 g
C) 5.67 g
D) 11.3 g
E) 102 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: The molar mass of silver is 47
Q57: 4.00 moles of sodium have a mass
Q88: What is the mass,in grams,of 1.00 mole
Q99: Given the following equation,what is the correct
Q100: The following is a double replacement reaction.<br>2
Q102: The following reaction is an example of
Q104: How many moles of carbon atoms are
Q107: One mole of copper(II)sulfate,CuSO<sub>4</sub>,contains _ moles of
Q108: In the following reaction,20.0 g of Al
Q109: One mole of neon atoms has a