Multiple Choice
For the following question(s) ,consider the following balanced equation. 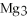
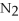 + 6
+ 6 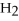 O → 3
O → 3 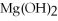 + 2
+ 2 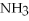
-How many grams of 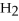 O are needed to produce 150 g of
O are needed to produce 150 g of 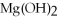 ?
?
A) 46 g
B) 18 g
C) 130 g
D) 93 g
E) 23 g
Correct Answer:

Verified
Correct Answer:
Verified
Q11: The molar mass of potassium is _.<br>A)19.00
Q12: The following reaction is correctly balanced.<br>2 <img
Q13: 3.00 moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="3.00 moles
Q16: Calculate the molar mass of magnesium chloride,MgCl<sub>2</sub>
Q16: For the following question(s),consider the following balanced
Q17: One mole of copper(II)sulfate,CuS <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="One
Q19: One mole of particles of any substance
Q20: What is the coefficient of hydrogen, <img
Q32: The molar mass of copper(II)chloride is 134.5
Q62: Answer the question(s)below about the following reaction.<br>2