Multiple Choice
For the following question(s) ,consider the following balanced equation. 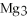
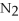 + 6
+ 6 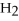 O → 3
O → 3 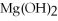 + 2
+ 2 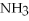
-Find the mass of 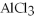 that is produced when 25.0 grams of
that is produced when 25.0 grams of 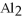
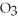 reacts with HCl according to the following equation.
reacts with HCl according to the following equation. 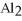
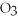 + 6HCl → 2
+ 6HCl → 2 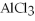 + 3
+ 3 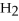 O
O
A) 155 g
B) 72.9 g
C) 65.3 g
D) 32.6 g
E) 16.3 g
Correct Answer:

Verified
Correct Answer:
Verified
Q11: The molar mass of potassium is _.<br>A)19.00
Q12: The following reaction is correctly balanced.<br>2 <img
Q13: 3.00 moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="3.00 moles
Q15: For the following question(s),consider the following balanced
Q16: Calculate the molar mass of magnesium chloride,MgCl<sub>2</sub>
Q17: One mole of copper(II)sulfate,CuS <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="One
Q19: One mole of particles of any substance
Q20: What is the coefficient of hydrogen, <img
Q21: 0.400 mole of sucrose, C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>,contains _ C
Q32: The molar mass of copper(II)chloride is 134.5