Multiple Choice
Answer the question(s) below about the following reaction.
2 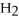
 → 2
→ 2 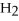 O +
O + 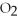
-How many moles of oxygen gas can 5.0 moles of hydrogen peroxide (H2O2) produce,if decomposition is complete?
A) 5.0 moles
B) 2.5 moles
C) 1.0 mole
D) 2.0 moles
E) 10.moles
Correct Answer:

Verified
Correct Answer:
Verified
Q17: In the reaction of hydrogen gas with
Q36: A reaction that releases energy as it
Q49: The following reaction is correctly balanced.<br>KCl <img
Q50: The following is a decomposition reaction.<br>2KClO<sub>3</sub> →
Q51: For the following question(s),consider the following balanced
Q52: The following reaction takes place when an
Q53: Acetylene gas, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="Acetylene gas,
Q56: What is the molar mass of <img
Q56: Avogadro's number is the number of grams
Q92: The _ is the energy difference between