Short Answer
Acetylene gas, 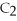
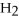 ,reacts with oxygen according to the following equation.If 1 mole of acetylene react completely with sufficient oxygen,how many moles of carbon dioxide are produced?
,reacts with oxygen according to the following equation.If 1 mole of acetylene react completely with sufficient oxygen,how many moles of carbon dioxide are produced?
2 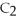
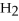 + 5
+ 5 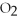 → 4C
→ 4C 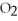 +
+  O
O
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: In the reaction of hydrogen gas with
Q36: A reaction that releases energy as it
Q49: The following reaction is correctly balanced.<br>KCl <img
Q50: The following is a decomposition reaction.<br>2KClO<sub>3</sub> →
Q51: For the following question(s),consider the following balanced
Q52: The following reaction takes place when an
Q54: Answer the question(s)below about the following reaction.<br>2
Q56: What is the molar mass of <img
Q92: The _ is the energy difference between
Q101: The _ is the minimum energy needed