Matching
Identify each of the following compounds as an acid,a base,or neither.
Premises:
NaN 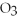
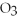
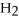
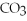
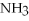
HCl
NaCl
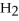
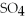
KOH
NaOH
Responses:
neither
acid
base
Correct Answer:
Premises:
Responses:
NaN 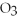
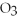
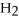
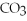
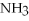
HCl
NaCl
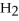
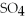
KOH
NaOH
Premises:
NaN 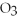
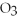
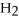
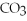
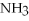
HCl
NaCl
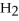
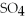
KOH
NaOH
Responses:
Related Questions
Q22: A buffer is a solution that tends
Q25: For <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="For ,the
Q26: According to LeChâtelier's principle,predict whether adding <img
Q26: A system is at equilibrium when the
Q27: In a neutralization reaction _.<br>A)two acids react
Q28: The [ H<sub>3</sub>O<sup>+</sup> ] of a solution
Q31: The neutralization reaction between <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="The
Q39: For many reactions of acids with bases,the
Q45: The function of a buffer is to
Q63: Ammonium hydroxide is a weak base because