Multiple Choice
According to the Brønsted-Lowry definition,________.
A) an acid is a proton acceptor
B) a base produces 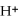 ions in aqueous solutions
ions in aqueous solutions
C) a base is a proton donor
D) a base is a proton acceptor
E) an acid acts as the solvent
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: The name given to an aqueous solution
Q12: HBr is a strong acid.
Q23: When an acid reacts with a metal
Q56: What is the pH of a solution
Q57: Which of the following is correctly identified?<br>A)
Q57: Normal blood pH is about _.<br>A)6.8<br>B)7.0<br>C)7.2<br>D)7.4<br>E)7.6
Q60: The [ H<sub>3</sub>O<sup>+</sup> ] of a solution
Q61: Which one of the following is characteristic
Q63: In a buffer system of HF and
Q67: If lung problems like emphysema occur,the blood