Multiple Choice
In a buffer system of HF and its salt,NaF,________.
A) the HF neutralizes added acid
B) the HF neutralizes added base
C) the HF is not necessary
D) the 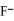 neutralizes added H2O
neutralizes added H2O
E) the 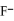 neutralizes added base
neutralizes added base
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: The name given to an aqueous solution
Q56: What is the pH of a solution
Q57: Normal blood pH is about _.<br>A)6.8<br>B)7.0<br>C)7.2<br>D)7.4<br>E)7.6
Q57: Which of the following is correctly identified?<br>A)
Q58: According to the Brønsted-Lowry definition,_.<br>A)an acid is
Q60: The [ H<sub>3</sub>O<sup>+</sup> ] of a solution
Q61: Which one of the following is characteristic
Q64: Which of the following could be a
Q65: The correct formula for sulfuric acid is
Q67: If lung problems like emphysema occur,the blood