Multiple Choice
What is the molecular formula of a compound given the molar mass of the compound is 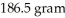 and the empirical formula is C2H7?
and the empirical formula is C2H7?
A) C4H14
B) C3H21
C) C2H7
D) C2H14
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: What is the value of n when
Q33: Water is 11.2% hydrogen by mass.
Q34: The simplest formula for hydrogen peroxide is
Q35: What is the mass in grams of
Q36: Determine the correct empirical formula of a
Q38: The empirical formula of a compound:<br>A)describes the
Q39: How many moles of Cu are in
Q40: What is the mass percent of Na
Q41: How many moles are there in 82.5
Q42: How many moles of fluorine are in