Multiple Choice
What is the value of n when the empirical formula is C3H5 and the molecular mass is 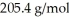 ?
?
A) 0.02
B) 5
C) 10
D) 140
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: What is the correct value for Avogadro's
Q28: If you have <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6110/.jpg" alt="If you
Q29: What is the mass percent of chlorine
Q30: Given that sodium chloride is 39.0% sodium
Q31: What is the mass of 3.09 ×
Q33: Water is 11.2% hydrogen by mass.
Q34: The simplest formula for hydrogen peroxide is
Q35: What is the mass in grams of
Q36: Determine the correct empirical formula of a
Q37: What is the molecular formula of a