Multiple Choice
How many grams of the excess reactant remain assuming the reaction goes to completion and that you start with 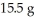 of Na2S and
of Na2S and  CuSO4?
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) 0.05
B) 15.45
C) 9.58
D) 5.92
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: What is the theoretical yield of waffles
Q32: The theoretical yield of a reaction is
Q33: What is the limiting reactant for the
Q34: Given that 4 NH<sub>3</sub> + 5 O<sub>2</sub>
Q35: What is the theoretical yield of a
Q37: How many grams of NO<sub>2</sub> are theoretically
Q38: Determine the theoretical yield of C when
Q39: How many moles of water are needed
Q40: If the theoretical yield of the reaction
Q41: The limiting reactant is not necessarily the