Multiple Choice
If the theoretical yield of the reaction below corresponds to 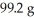 and the actual yield was 60.9 g,calculate the percent yield.
and the actual yield was 60.9 g,calculate the percent yield.
Given: Li2O + H2O → 2 LiOH
A) 16.0 %
B) 71.8 %
C) 38.0 %
D) 61.4 %
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: What is the theoretical yield of a
Q36: How many grams of the excess reactant
Q37: How many grams of NO<sub>2</sub> are theoretically
Q38: Determine the theoretical yield of C when
Q39: How many moles of water are needed
Q41: The limiting reactant is not necessarily the
Q42: Given the recipe: 2 cups flour +
Q43: What is the excess reactant for the
Q44: Consider a reaction of chemicals depicted as
Q45: The limiting reactant is the product that