Multiple Choice
What is the concentration of the hydronium ions in a neutral solution?
A) 0.0 M
B) 1.0 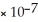 M
M
C) 1.0 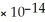 M
M
D) > 1.0 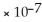 M
M
E) < 1.0 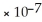 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: A neutral solution does not contain any
Q33: What is the pH of a solution
Q34: Since diprotic acids such as H<sub>2</sub>CO<sub>3</sub> have
Q35: What are the products of a neutralization
Q36: A simple buffer can be prepared by
Q38: When a metal reacts with an acid
Q39: H⁺ is called the hydronium ion.
Q40: In the following reaction: NH<sub>4</sub><sup>+ </sup>(aq)+ H<sub>2</sub>O
Q41: Which of the following contributes to acid
Q42: Pure water cannot conduct electricity so why