Multiple Choice
What is the pH of a solution that has a H⁺ concentration equal to 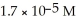 ?
?
A) 4.77
B) 5.20
C) 0.22
D) 10.20
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Sodium hydroxide is often used in the
Q29: Consider a 1.6 × 10<sup>-3</sup> M solution
Q30: In order for a solution to be
Q31: What is the concentration of H<sup>+</sup> in
Q32: A neutral solution does not contain any
Q34: Since diprotic acids such as H<sub>2</sub>CO<sub>3</sub> have
Q35: What are the products of a neutralization
Q36: A simple buffer can be prepared by
Q37: What is the concentration of the hydronium
Q38: When a metal reacts with an acid