Multiple Choice
Identify the substance being reduced in the following reaction: 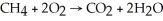 .
.
A) CH4
B) O2
C) CO2
D) H2O
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Which battery system is completely rechargeable?<br>A)alkaline batteries<br>B)fuel
Q32: Given that the Activity Series shown below
Q33: The reducing agent is reduced during the
Q34: In the following reaction, Zn (s)+ CuSO<sub>4</sub>
Q35: The rusting of iron is an example
Q37: Corrosion can be defined as the reduction
Q38: From the activity list included in this
Q39: What is the oxidation state of nitrogen
Q40: Examine the activity list given below to
Q41: Assign the oxidation state of each atom