Multiple Choice
In the following reaction, Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s) :
A) Zn is the reducing agent and CuSO4 is the oxidizing agent.
B) CuSO4 is the reducing agent and Zn is the oxidizing agent.
C) ZnSO4 is the reducing agent and Cu is the oxidizing agent.
D) 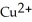 is the reducing agent and Zn2+ is the oxidizing agent.
is the reducing agent and Zn2+ is the oxidizing agent.
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q29: In which compound does manganese have the
Q30: Corrosion can be prevented by all of
Q31: Which battery system is completely rechargeable?<br>A)alkaline batteries<br>B)fuel
Q32: Given that the Activity Series shown below
Q33: The reducing agent is reduced during the
Q35: The rusting of iron is an example
Q36: Identify the substance being reduced in the
Q37: Corrosion can be defined as the reduction
Q38: From the activity list included in this
Q39: What is the oxidation state of nitrogen