Multiple Choice
What is the oxidation state of the underlined atom in the reaction: 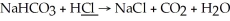
A) 0
B) +1
C) -1
D) +2
E) -2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q103: If you properly balance the following half
Q104: In the following reaction, Mg (s)+ <img
Q105: Reduction can be defined as the gain
Q106: Reduction involves which of the following? <br>1.Loss
Q107: Suggest two methods to reduce corrosion of
Q109: What is the balanced oxidation half-reaction for
Q110: For the reaction KMnO<sub>4</sub> + Li →
Q111: Reduction typically involves:<br>A)the loss of electrons.<br>B)the gain
Q112: The oxidation number of sulfur in SO<sub>3</sub>
Q113: The oxidation number of sodium in NaI