Multiple Choice
In the following reaction, Mg (s) + 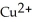 (aq) →
(aq) → 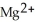 (aq) + Cu (s) :
(aq) + Cu (s) :
A) Mg is the reducing agent and Cu is the oxidizing agent.
B) 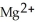 is the reducing agent and Cu is the oxidizing agent.
is the reducing agent and Cu is the oxidizing agent.
C) Cu is the reducing agent and 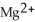 is the oxidizing agent.
is the oxidizing agent.
D) 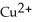 is the reducing agent and Mg is the oxidizing agent.
is the reducing agent and Mg is the oxidizing agent.
E) Mg is the reducing agent and 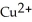 is the oxidizing agent.
is the oxidizing agent.
Correct Answer:

Verified
Correct Answer:
Verified
Q99: How many electrons are exchanged when the
Q100: Balance the redox reaction in acid solution:
Q101: Cars are being developed that run on
Q102: Electrolysis is used to recover many metals
Q103: If you properly balance the following half
Q105: Reduction can be defined as the gain
Q106: Reduction involves which of the following? <br>1.Loss
Q107: Suggest two methods to reduce corrosion of
Q108: What is the oxidation state of the
Q109: What is the balanced oxidation half-reaction for