Multiple Choice
From the activity list included in this problem,which element/ion will spontaneously react with Ca?
Activity Series = 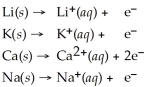
A) Na
B) 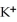
C) 
D) Li
E) 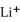
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: Using the activity list included in this
Q86: The half-reaction 2 Br <sup>1-</sup> → Br<sub>2</sub>
Q87: The oxidation state of Na in Na<sub>2</sub>SO<sub>4</sub>
Q88: Oxidation and reduction cannot both occur in
Q89: Electrical current can be used to drive
Q91: The reducing agent typically:<br>A)gains electrons.<br>B)always remains unchanged
Q92: Reactions involving the transfer of electrons are
Q93: Corrosion is the:<br>A)oxidation of nonmetals.<br>B)loss of electrons.<br>C)oxidation
Q94: A galvanic cell is a spontaneous electrochemical
Q95: What is the oxidation state of the