Multiple Choice
What is the oxidation state of the underlined atom in the reaction: 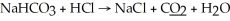
A) 0
B) +1
C) -1
D) +2
E) -2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: From the activity list included in this
Q91: The reducing agent typically:<br>A)gains electrons.<br>B)always remains unchanged
Q92: Reactions involving the transfer of electrons are
Q93: Corrosion is the:<br>A)oxidation of nonmetals.<br>B)loss of electrons.<br>C)oxidation
Q94: A galvanic cell is a spontaneous electrochemical
Q96: Identify the reducing agent in the following
Q97: Oxidation is the loss of electrons.
Q98: Which substance below would contain a nitrogen
Q99: How many electrons are exchanged when the
Q100: Balance the redox reaction in acid solution: