Multiple Choice
A Lewis structure of boron trifluoride is shown here. How many valence electrons are shown directly around the B atom 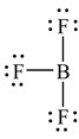
A) Eight
B) Six
C) Twenty-four
D) None
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Calculate the energy change for the
Q19: Shown here is a Lewis structure for
Q20: A polar covalent bond would form in
Q21: The compound Al(ClO<sub>3</sub>)<sub>3</sub> shows only ionic bonding.
Q22: What is the total number of lone
Q24: Which one of these polar covalent bonds
Q25: Assuming the octet rule is obeyed, how
Q26: The Lewis structure shown here is correct
Q27: Which of the following ionic solids would
Q28: The properties and chemical reactivity of a