Multiple Choice
Shown here is a Lewis structure for the chlorate ion, ClO3-, that expands the octet to minimize formal charge and if necessary places negative formal charges on the most electronegative atom(s) . What is the formal charge on Cl 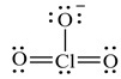
A) +1
B) -1
C) 0
D) +2
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The Lewis structure reveals only single bonds
Q15: The Lewis structure for the nitrate ion,
Q16: Which one of the following compounds utilizes
Q17: A Lewis structure for SO<sub>3</sub> that obeys
Q18: Calculate the energy change for the
Q20: A polar covalent bond would form in
Q21: The compound Al(ClO<sub>3</sub>)<sub>3</sub> shows only ionic bonding.
Q22: What is the total number of lone
Q23: A Lewis structure of boron trifluoride is
Q24: Which one of these polar covalent bonds