Multiple Choice
Determine if each of the four processes below describes positive or negative changes to the internal energy of the system.
I. water absorbs heat from the surroundings and becomes steam
II. steam expands and pushes against the surrounding air
III. fuel molecules burn and heat the surroundings
IV. air is compressed into an inner tube by an external pump
A) 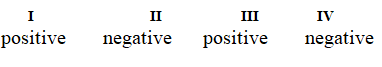
B) 
C) 
D) 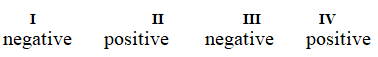
E) 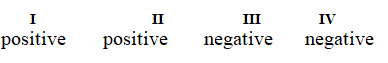
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Which of the following statements is false?<br>A)
Q22: What is the molar heat capacity of
Q23: The standard enthalpies of formation for several
Q24: Which of the following is an example
Q25: What is the enthalpy change for the
Q27: How much energy in kilojoules is required
Q28: Which process is exothermic?<br>A) freezing rain drops<br>B)
Q29: A sample of water containing 2.00 moles
Q30: The temperature of 3.50 kg of water
Q31: What is the enthalpy change when 22.5