Multiple Choice
The standard enthalpies of formation for several substances are given below:  Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.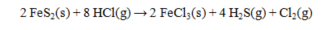
A) -881.4 kJ
B) -811.8 kJ
C) -149.6 kJ
D) +149.6 kJ
E) +213.4 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: Determine the amount of heat required to
Q19: The complete combustion of 1.47 g of
Q20: Based on the following thermochemical equation below,
Q21: Which of the following statements is false?<br>A)
Q22: What is the molar heat capacity of
Q24: Which of the following is an example
Q25: What is the enthalpy change for the
Q26: Determine if each of the four processes
Q27: How much energy in kilojoules is required
Q28: Which process is exothermic?<br>A) freezing rain drops<br>B)