Multiple Choice
The complete combustion of 1.47 g of methanol produces 29.3 kJ of heat. Determine the DH° for the reaction and its sign. 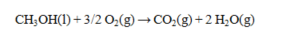
A) -938 kJ
B) -638 kJ
C) -1.35 kJ
D) +638 kJ
E) +938 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: How much energy is required to melt
Q15: A 20.0 g sample of aluminum (specific
Q16: Which statement about energy is false?<br>A) Thermodynamics
Q17: Based on the following thermochemical equation, which
Q18: Determine the amount of heat required to
Q20: Based on the following thermochemical equation below,
Q21: Which of the following statements is false?<br>A)
Q22: What is the molar heat capacity of
Q23: The standard enthalpies of formation for several
Q24: Which of the following is an example