Multiple Choice
Which statement about the reaction below is true, given large amounts of reactants? 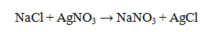
A) NaNO3 is insoluble in water and will precipitate.
B) AgCl is insoluble in water and will precipitate.
C) Both NaNO3 and AgCl are insoluble in water and will precipitate.
D) AgNO3 is insoluble in water and no reaction will occur.
E) All compounds in the reaction are soluble in water and no reaction occurs.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: A 50.0 mL sample of 0.108 M
Q2: Which of the following is a reducing
Q3: Determine the mass of BaSO<sub>4</sub> that is
Q5: A 65.00 mL sample of HNO<sub>3</sub> solution
Q6: A 64.05 mL sample of 0.250 M
Q7: What is reduced in the reaction below?
Q8: Which statement about strong acids is true?<br>A)
Q9: Which statement about bases is true?<br>A) Bases
Q10: Which substance is reduced in the reaction
Q11: Determine the ammonium ion concentration of a