Multiple Choice
Determine the mass of BaSO4 that is produced by the reaction of 45.0 mL of 0.155 M H2SO4 and 60.0 mL of 0.125 M BaCl2. Assume that BaSO4 is totally insoluble. 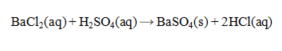
A) 1.45 g
B) 1.62 g
C) 1.79 g
D) 3.24 g
E) 0.775 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A 50.0 mL sample of 0.108 M
Q2: Which of the following is a reducing
Q4: Which statement about the reaction below is
Q5: A 65.00 mL sample of HNO<sub>3</sub> solution
Q6: A 64.05 mL sample of 0.250 M
Q7: What is reduced in the reaction below?
Q8: Which statement about strong acids is true?<br>A)
Q9: Which statement about bases is true?<br>A) Bases
Q10: Which substance is reduced in the reaction
Q11: Determine the ammonium ion concentration of a