Multiple Choice
Hydrochloric acid solutions are often standardized by the reaction below. How many grams of CaCO3 are required to exactly react with 50.0 mL of 0.155 M HCl? 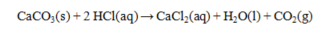
A) 0.194 g
B) 0.283 g
C) 0.341 g
D) 0.387 g
E) 0.566 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Which substance is reduced in the reaction
Q11: Determine the ammonium ion concentration of a
Q12: What is the oxidation number of N
Q13: Oxalic acid, H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>, reacts with Y(NO<sub>3</sub>)<sub>3</sub> as
Q14: What is the oxidation number of O
Q16: Which reaction will not occur?<br>A) Al(s) +
Q17: Which of the methods described below will
Q18: Which one or two substances are reduced
Q19: How many moles of ions are in
Q20: Which substance is an oxidizing agent in