Multiple Choice
Oxalic acid, H2C2O4, reacts with Y(NO3) 3 as shown by the equation below. What weight of yttrium oxalate is produced from 50.0 mL of 0.265 M Y(NO3) 3 and excess oxalic acid? Assume that all of the yttrium oxalate is insoluble. 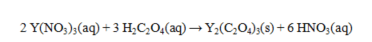
A) 1.46 g
B) 1.82 g
C) 2.93 g
D) 3.64 g
E) 5.85 g
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which statement about strong acids is true?<br>A)
Q9: Which statement about bases is true?<br>A) Bases
Q10: Which substance is reduced in the reaction
Q11: Determine the ammonium ion concentration of a
Q12: What is the oxidation number of N
Q14: What is the oxidation number of O
Q15: Hydrochloric acid solutions are often standardized by
Q16: Which reaction will not occur?<br>A) Al(s) +
Q17: Which of the methods described below will
Q18: Which one or two substances are reduced