Multiple Choice
How many moles of H2O are formed from the complete combustion of 45.0 g of methanol, CH3OH? 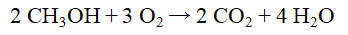
A) 0.711 mol
B) 1.41 mol
C) 1.42 mol
D) 2.81 mol
E) 50.6 mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: The Roman numerals in the reaction given
Q56: If 935 g of cesium reacts with
Q57: Which statement about the expression 6 C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>
Q58: The Roman numerals in the reaction given
Q59: For the reaction given below, how many
Q60: The Roman numerals in the reaction given
Q61: In the reaction given below, how many
Q63: Match the following:
Q64: If 110.0 g of iron reacts with
Q65: In the reaction given below, for every