Multiple Choice
If 110.0 g of iron reacts with 64.0 g of oxygen, what is the theoretical yield of Fe2O3? 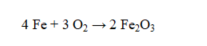
A) 157.3 g
B) 212.9 g
C) 314.9 g
D) 319.4 g
E) 629.2 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: The Roman numerals in the reaction given
Q56: If 935 g of cesium reacts with
Q57: Which statement about the expression 6 C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>
Q58: The Roman numerals in the reaction given
Q59: For the reaction given below, how many
Q60: The Roman numerals in the reaction given
Q61: In the reaction given below, how many
Q62: How many moles of H<sub>2</sub>O are formed
Q63: Match the following:
Q65: In the reaction given below, for every