Multiple Choice
In the reaction given below, how many grams of C14H9Cl5 will be produced by the reaction of 25.0 g of each of the starting materials? 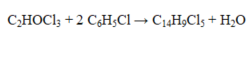
A) 39.4 g
B) 78.7 g
C) 121 g
D) 157 g
E) 354 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Classify the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Classify
Q34: How many mol of ammonia will be
Q35: The complete reaction of 16.12 g of
Q36: How many grams of Al<sub>2</sub>O<sub>3</sub> are formed
Q37: In the reaction given, if 25.0 g
Q39: How many grams of HCl are required
Q40: How many grams of PH<sub>3</sub> are formed
Q41: In the reaction shown below, which substances
Q42: The efficiency of a particular synthesis method
Q43: If 32.0 g of oxygen reacts with