Multiple Choice
If 32.0 g of oxygen reacts with sufficient magnesium to produce magnesium oxide, what is the theoretical yield? 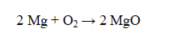
A) 20.2 g
B) 32.0 g
C) 40.3 g
D) 80.6 g
E) 161 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: In the reaction given below, how many
Q39: How many grams of HCl are required
Q40: How many grams of PH<sub>3</sub> are formed
Q41: In the reaction shown below, which substances
Q42: The efficiency of a particular synthesis method
Q44: Which statement regarding the complete combustion of
Q45: Classify the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Classify
Q46: The reaction below is an example of
Q47: How many grams of Na<sub>2</sub>O are formed
Q48: What is the percent yield when 0.750