Multiple Choice
Select the material containing the element with the highest oxidation number and determine how many electrons are required to reduce that element to an oxidation number of zero. 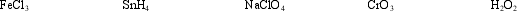
A) 8
B) 7
C) 6
D) 4
E) 3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Exhibit 18-2 Use this list of
Q5: Answer the following questions:<br>a. Briefly explain why
Q6: Which statement concerning the number of electrons
Q7: Explain why galvanizing steel chain-link fencing is
Q8: Exhibit 18-2 Use this list of half-reactions
Q10: Which process could not be an electrolytic
Q11: Which of these components must be present
Q12: An underground steel fuel pipe buried in
Q13: The value of E<sup> <span class="ql-formula"
Q14: Which statement is correct?<br>A) all electrolytic cells