Multiple Choice
The value of E cell for the cell shown below is + 1.41 V. 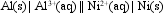
What is the value of Ecell at 25 C if the concentration of Al3+(aq) is 0.050 M, and of Ni2+(aq) , 2.0 M?
A) +1.34 V
B) +1.38 V
C) +1.41 V
D) +1.44 V
E) +1.48 V
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Exhibit 18-2 Use this list of half-reactions
Q9: Select the material containing the element with
Q10: Which process could not be an electrolytic
Q11: Which of these components must be present
Q12: An underground steel fuel pipe buried in
Q14: Which statement is correct?<br>A) all electrolytic cells
Q15: The value of E<sub>cell</sub> at 25<sup>
Q16: Refer to the following values of standard
Q17: Which statement concerning the proton exchange membrane
Q18: Which change does not indicate a reduction?<br>A)