Multiple Choice
Consider an electrochemical cell as shown, with Zn in ZnCl2(aq) and Cu in Cu(NO3) 2(aq) , and a salt bridge containing KNO3(aq) . The overall chemical reaction is 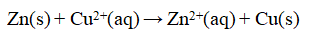
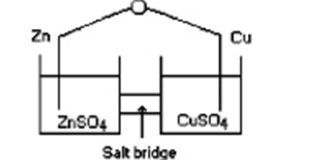
Which statement is correct?
A) one mole of electrons is transferred in this reaction
B) copper is oxidized at the anode
C) electrons travel from the Zn electrode to the Cu electrode
D) this is an example of a concentration cell
E) zinc is reduced at the cathode
Correct Answer:

Verified
Correct Answer:
Verified
Q27: In the anode compartment of a simple
Q28: Consider the cell reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg"
Q29: When the reaction shown below is balanced,
Q30: Which change describes an oxidation half-reaction?<br>A) decrease
Q31: Unwanted oxidation of a metal exposed to
Q33: If all of the following metals
Q34: Which statement is not correct?<br>A) an electrochemical
Q35: Calculate the mass of cobalt that will
Q36: The value of E<sup> <span class="ql-formula"
Q37: Galvanizing a metal (coating its surface with