Multiple Choice
Consider the cell reaction 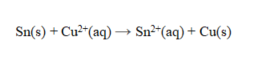 The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.
The value of E cell is 0.447 V at 25 C. Calculate the value of DG and K for this cell.
A) -86.3 kJ; 1.26 × 1015
B) -43.1 kJ; 1.37 × 1043
C) 43.1 kJ; 3.55 × 107
D) 86.3 kJ; 7.92 × 10-16
E) 86.3 kJ; 2.00 × 1086
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: Which cell notation represents a battery constructed
Q24: The unit used to measure electromotive force
Q25: Is the part of a flashlight battery
Q26: Which of the following would require the
Q27: In the anode compartment of a simple
Q29: When the reaction shown below is balanced,
Q30: Which change describes an oxidation half-reaction?<br>A) decrease
Q31: Unwanted oxidation of a metal exposed to
Q32: Consider an electrochemical cell as shown, with
Q33: If all of the following metals