Multiple Choice
The freezing points of the following aqueous solutions, from highest to lowest, are: 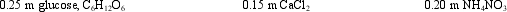
A) C6H12O6 > NH4NO3 > CaCl2
B) C6H12O6 > CaCl2 > NH4NO3
C) CaCl2 > C6H12O6 > NH4NO3
D) CaCl2 > NH4NO3 > C6H12O6
E) NH4NO3 > C6H12O6 > CaCl2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: The process of dissolving is favored if
Q48: The _ of a substance is defined
Q49: Osmotic pressure is the<br>A) pressure that must
Q50: Molality (m) is defined as moles of
Q51: A concentrated antifreeze solution contains 580. g
Q53: For inorganic compounds, solubility in water is<br>A)
Q54: An aqueous solution of phosphoric acid, H<sub>3</sub>PO<sub>4</sub>,
Q55: Calculate the boiling point of a
Q56: A solution with solute concentration equal to
Q57: The concentration unit one part per billion