Essay
An aqueous solution of phosphoric acid, H3PO4, contains 285 g H3PO4 in 400 mL solution, and has a density of 1.35 g/mL. Calculate
a. the weight % 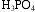 in this solution.
in this solution.
b. The concentration in mol/L of this solution.
Correct Answer:

Verified
a. Total mass of solution = volume ' den...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q47: The process of dissolving is favored if
Q48: The _ of a substance is defined
Q49: Osmotic pressure is the<br>A) pressure that must
Q50: Molality (m) is defined as moles of
Q51: A concentrated antifreeze solution contains 580. g
Q52: The freezing points of the following aqueous
Q53: For inorganic compounds, solubility in water is<br>A)
Q55: Calculate the boiling point of a
Q56: A solution with solute concentration equal to
Q57: The concentration unit one part per billion