Multiple Choice
The equilibrium constant expression for the reaction shown below is 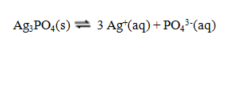
A) 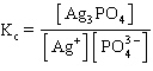
B) 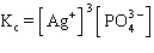
C) 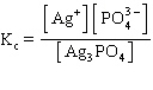
D) 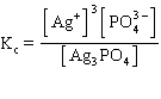
E) 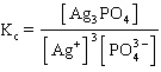
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The concentration equilibrium constant, K<sub>c</sub>, and the
Q15: In predicting which side of an equilibrium
Q16: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider the
Q17: Consider the endothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q18: Consider the equilibrium system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q20: The equilibrium constant for a reaction is
Q21: Once the reaction quotient, Q, has been
Q22: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q23: If the equilibrium constants for the two
Q24: Concerning the Haber-Bosch for the synthesis of