Multiple Choice
Consider the reaction 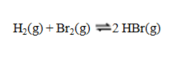 If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
A) 3.5 × 104
B) 1.4 × 104
C) 6.8 × 103
D) 3.7 × 102
E) 1.9 × 102
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Consider the exothermic reaction at equilibrium: <img
Q12: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider the
Q13: Considering only the probability factor in the
Q14: The concentration equilibrium constant, K<sub>c</sub>, and the
Q15: In predicting which side of an equilibrium
Q17: Consider the endothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q18: Consider the equilibrium system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q19: The equilibrium constant expression for the reaction
Q20: The equilibrium constant for a reaction is
Q21: Once the reaction quotient, Q, has been