Multiple Choice
Consider the reaction 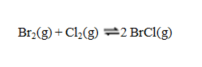 If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature is
A) 6.99
B) 4.70
C) 1.91
D) 0.532
E) 0.142
Correct Answer:

Verified
Correct Answer:
Verified
Q25: The reaction<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="The reaction
Q26: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q27: This reaction is the basis for the
Q28: For a particular chemical reaction, which of
Q29: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q31: A chemical equilibrium may involve<br>A) reactants and
Q32: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider the
Q33: Which statement concerning product-favored reactions is not
Q34: If a reaction is product-favored on the
Q35: A weak acid is 5% ionized at