Multiple Choice
This reaction is the basis for the Haber-Bosch process for the manufacture of ammonia: 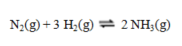 To maximize the yield of ammonia, the pressure ____ and the temperature____.
To maximize the yield of ammonia, the pressure ____ and the temperature____.
A) should be kept high by compressing the mixture; should be kept low
B) should be kept high by compressing the mixture; should be kept high
C) should be kept high by adding an inert gas; should be kept low
D) should be kept high by adding an inert gas; should be kept high
E) should be kept low; should be kept low
Correct Answer:

Verified
Correct Answer:
Verified
Q22: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q23: If the equilibrium constants for the two
Q24: Concerning the Haber-Bosch for the synthesis of
Q25: The reaction<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="The reaction
Q26: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q28: For a particular chemical reaction, which of
Q29: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q30: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider the
Q31: A chemical equilibrium may involve<br>A) reactants and
Q32: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider the