Multiple Choice
For the reaction 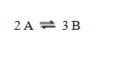 Kc = 1.37. If the concentrations of A and B are equal, what is the value of that concentration?
Kc = 1.37. If the concentrations of A and B are equal, what is the value of that concentration?
A) 0.685 M
B) 0.822 M
C) 1.17 M
D) 1.37 M
E) 1.88 M
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Consider the equilibrium system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q42: In a balanced chemical equation, if there
Q43: If the reaction quotient, Q, is greater
Q44: Which of the following is not true
Q45: For an exothermic reaction, an increase in
Q47: If the value of K<sub>c</sub> for a
Q48: The equilibrium for a particular chemical reaction
Q49: Consider the reaction<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="
Q50: Consider the equilibrium reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q51: Consider the gas-phase equilibrium A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg"