Essay
Consider the reaction
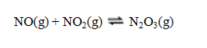 for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
The expression for 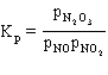 .
.
Correct Answer:

Verified
Set up the reaction table as described i...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q44: Which of the following is not true
Q45: For an exothermic reaction, an increase in
Q46: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="For the
Q47: If the value of K<sub>c</sub> for a
Q48: The equilibrium for a particular chemical reaction
Q50: Consider the equilibrium reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q51: Consider the gas-phase equilibrium A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg"
Q52: Which of the following is false?<br>A) a
Q53: Which of the following is true for
Q54: A particular reaction mixture (K<sub>p</sub> = 10)