Multiple Choice
The decomposition of N2O(g) to nitrogen and oxygen has the rate law 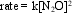 where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
A) 9.5 × 10-3 M
B) 2.2 × 10-3 M
C) 0.029 M
D) 0.17 M
E) 0.46 M
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which statement, comparing catalyzed and uncatalyzed versions
Q3: Consider this proposed mechanism for the reaction
Q4: A certain reaction is studied at room
Q5: Which of the following factors will affect
Q6: The activation energy in the Arrhenius equation
Q7: Which of the following factors will affect
Q8: Which unit is appropriate for describing
Q9: The rate law for a given reaction
Q10: If the rate law for an elementary
Q11: For a reaction that is zeroth-order with