Multiple Choice
Arrange the following gaseous substances in order of decreasing average molecular speed at 25°C. 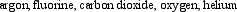
A) helium > oxygen > fluorine > carbon dioxide > argon
B) helium > oxygen > fluorine > argon > carbon dioxide
C) argon > oxygen > helium > carbon dioxide > fluorine
D) carbon dioxide > argon > fluorine > oxygen > helium
E) carbon dioxide > fluorine > argon > oxygen > helium
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Based on temperature, there are four main
Q3: Aluminum and oxygen react to form aluminum
Q4: If, at constant temperature, the volume of
Q5: When the external pressure on a balloon
Q6: Which statement summarizes Charles's Law?<br>A) The volume
Q7: What is the correct name for the
Q8: A mixture of He, Ne and Ar
Q9: If, at constant pressure, the absolute temperature
Q10: Calculate the number of moles of a
Q11: Derive the gas constant (in L torr