Multiple Choice
Assume that the mixture of substances in drawing (1) undergoes a chemical reaction.Which of the drawings (2) -(4) represents a product mixture that is consistent with the law of mass conservation? 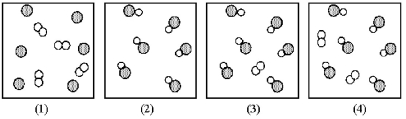
A) drawing (2)
B) drawing (3)
C) drawing (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q26: The chemical formula for calcium nitride is:<br>A)Ca(NO<sub>3</sub>)<sub>2</sub><br>B)Ca(NO<sub>2</sub>)<sub>2</sub><br>C)Ca<sub>3</sub>N<sub>2</sub><br>D)CaN<sub>2</sub>
Q34: As the atomic number of the elements
Q45: 24.0 g of which element contains the
Q47: Carbon dioxide is an example of<br>A)a compound.<br>B)an
Q80: Which one of the following compounds contains
Q110: The thiosulfate ion is<br>A)HS<sup>-</sup>.<br>B)HSO<sub>4</sub><sup>2-</sup>.<br>C)SO<sub>5</sub><sup>2-</sup>.<br>D)S<sub>2</sub>O<sub>3</sub><sup>2-</sup>.
Q121: Gold is an example of:<br>A)a compound<br>B)an element<br>C)a
Q140: The formula for dinitrogen trioxide is<br>A)N(OH)<sub>3.</sub><br>B)(NO<sub>3</sub>)<sub>2.</sub><br>C)N<sub>2</sub>O<sub>3.</sub><br>D)N<sub>3</sub>O<sub>2.</sub>
Q228: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q262: Use the periodic table below to answer